3 Balloons give a trigonal planar geometry. The IUPAC name of this compound is phenyl methanol.
 C6h6 Benzene Lewis Dot Structure And Polarity Science Struck
C6h6 Benzene Lewis Dot Structure And Polarity Science Struck
Drawing the Lewis Structure for CH 3 CN.
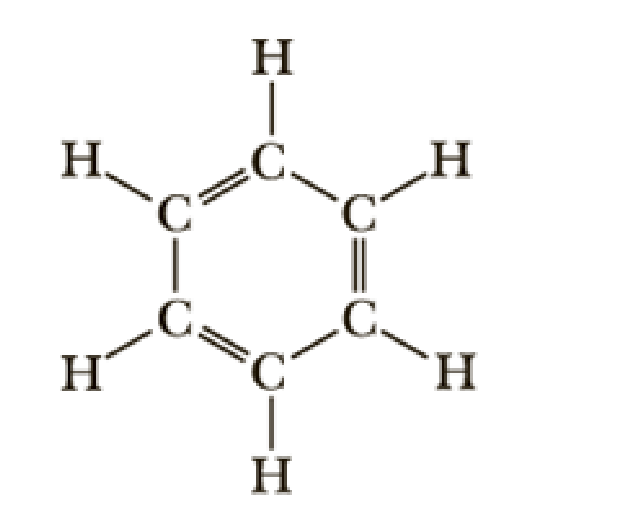
Hd c6h6 lewis structure and the description. In that they fill the outer shells of each atom in the structure and use the exact number of valence electrons available for the C6H6 Lewis structure. 100 0C 5 min 5 0Cmin -. Because it contains only carbon and hydrogen atoms benzene is classed as a hydrocarbon.
Well put 2 between each of the atoms to form chemical bonds. To accurately reflect the nature of the bonding benzene is. With CH 3 CN youll need a triple bond between the.
It is composed of 6 carbon atoms in a ring with 1 hydrogen atom attached to each carbon atom with the molecular formula c6h6. At room temperature benzyl alcohol exists as a colourless liquid that has a mildly aromatic smell. Phosphorus is the least electronegative--well put that in the center--and then we have 3 Chlorines.
Estradiol measurements are used in the diagnosis and treatment of various hormonal sexual disorders and in assessing placental function in complicated pregnancy. Each of the six carbon atoms is taken to be sp2 hybridized. 50 0C 2 0Cmin -.
When this aromatic alcohol is deprotonated the resulting anion is called a benzylate. With C6H6 there are sevearl possible Lewis structures that can be drawn. 2 Balloons give a linear geometry.
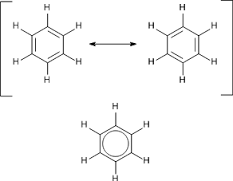
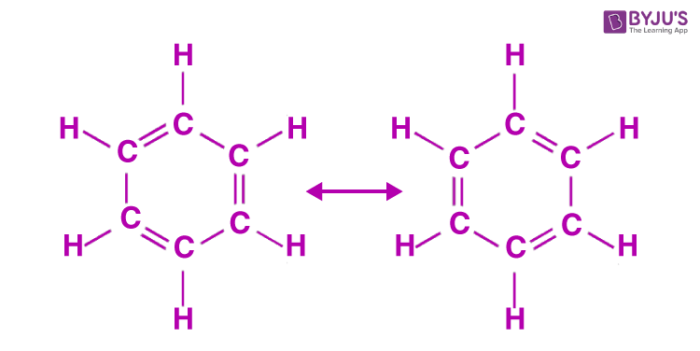
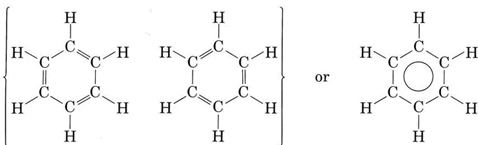
C6H6 has a total of 18 valence electrons. The description of the planar hexagonal benzene molecule C 6 H 6 illustrates another aspect of VB theory. The effect can be replicated by holding 2 3 4 5 or 6 balloons together.
Two of the hybrid orbitals are used to form σ bonds with the carbon atom neighbours and one is used to form a σ bond with a hydrogen atom. Benzene is an organic compound with the molecular formula C6H6. Lets put them around the Phosphorus and well put the Oxygen on top.
The most common Lewis structure for C6H6 is Benzene. The first structure is called cyclopropane. For Cl3PO we have a total of 32 valence electrons.
Lewis Dot Structure and Polarity. Scientists who were familiar with the chemical formula of benzene c6h6 were mystified about its molecular structure. So lets look at the two ways you can draw the C3H6 Lewis structure.
It is likely that this stability contributes to the peculiar molecular and chemical properties known as aromaticity. An estradiol test system is a device intended to measure estradiol an estrogenic steroid in plasma. Benzene is an organic chemical compound with the molecular formula C 6 H 6.
The CH 3 CN chemical formula gives you a strong hint that CH3 will be attached to the central atom. The benzene molecule is composed of six carbon atoms joined in a planar ring with one hydrogen atom attached to each. Carbon C is the least electronegative atom and goes at the center of the CH 3 CN Lewis structure.
This value is exactly halfway between the CC distance 134 and CC distance 146 of a CCCC unit suggesting a bond type midway between a double bond and a single bond all bond angles are. A step-by-step explanation of how to draw the XeF6 Lewis Structure. For the XeF6 Lewis structure we first count the valence electrons for the XeF6 molecu.
This is the C3H6 Lewis structure. When we see cyclo were thinking ring. In this ScienceStruck post we provide you with the polarity and steps to create the Lewis dot diagram of this aromatic compound.
The thing about C3H6 is theres more than one way to draw it based on the chemical formula that were given here. The molecular orbital description involves the formation of three delocalized π orbitals spanning all six carbon atoms while the valence bond description involves a superposition of resonance structures. This is the Cl3PO Lewis structure.
There are a total of 16 valence electrons in the CH 3 CN Lewis structure. A step-by-step explanation of how to draw the COCl2 Lewis Dot Structure PhosgeneFor the COCl2 structure use the periodic table to find the total number of. Benzyl alcohol is an organic compound with the chemical formula C 6 H 5 CH 2 OH.
06062021 Structure of benzene resonance contributors mesomeric structures pg6. The overall geometry of the atomic centre is determined by the mutual repulsion between the electron pairs of the total coordination number. Experimental studies especially those employing X-ray diffraction show benzene to have a planar structure with each carbon-carbon bond distance equal to 140 angstroms.
For C3H6 we have a total of 18 valence electrons. We have 32 valence electrons.
 Download C6h6 Mp3 Dan Mp4 2018 Otter Mp3
Download C6h6 Mp3 Dan Mp4 2018 Otter Mp3
 What Is The Resonance Structure For C 6h 6 Socratic
What Is The Resonance Structure For C 6h 6 Socratic
 C6h6 Benzene Lewis Dot Structure And Polarity Science Struck
C6h6 Benzene Lewis Dot Structure And Polarity Science Struck
What Is The Electron Dot Structure Of Benzene Edusaint Q A
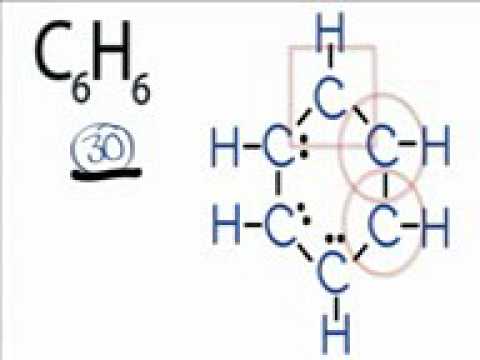 C6h6 Lewis Structure How To Draw The Lewis Structure For C6h6 Benzene Reg 22737 Youtube
C6h6 Lewis Structure How To Draw The Lewis Structure For C6h6 Benzene Reg 22737 Youtube
 What Is Benzene C6h6 Definition Discovery Structure Resonance Aromaticity Uses
What Is Benzene C6h6 Definition Discovery Structure Resonance Aromaticity Uses
 What Is The Resonance Structure For C 6h 6 Socratic
What Is The Resonance Structure For C 6h 6 Socratic
 Following Is A Structural Formula Of Benzene C 6 H 6 Which We Study In Chapter 21 A Using Vsepr Predict Each H C C And C C C Bond Angle In Benzene B State
Following Is A Structural Formula Of Benzene C 6 H 6 Which We Study In Chapter 21 A Using Vsepr Predict Each H C C And C C C Bond Angle In Benzene B State
What Is The Lewis Structure Of Benzene Quora
 Is Benzene Polar Or Non Polar Quora
Is Benzene Polar Or Non Polar Quora
 C6h6 Lewis Structure How To Draw The Lewis Structure For C6h6 Benzene Voicetube Learn English Through Videos
C6h6 Lewis Structure How To Draw The Lewis Structure For C6h6 Benzene Voicetube Learn English Through Videos
What Is The Lewis Structure Of Benzene Quora
 Benzene Lewis Structure Molecular Geometry Hybridization Polarity And Mo Diagram Techiescientist
Benzene Lewis Structure Molecular Geometry Hybridization Polarity And Mo Diagram Techiescientist
 Benzene Ring Structure Youtube
Benzene Ring Structure Youtube